Discuss the following types of corrosion
- Differential metallic corrosion
- Water line corrosion
Explanation
1414
0
Differential Metallic corrosion:
- When two dissimilar metals are in direct contact with one another and exposed to a corrosive medium, the metal with lower electrode potential becomes anode and suffers from corrosion, whereas the metal with higher electrode potential becomes cathode and protected from corrosion. This type of corrosion is known as differential metal corrosion or galvanic corrosion.
- The rate of corrosion depends mainly on the difference in the position of the two metals in galvanic series.
- Higher the difference, faster is the rate of corrosion
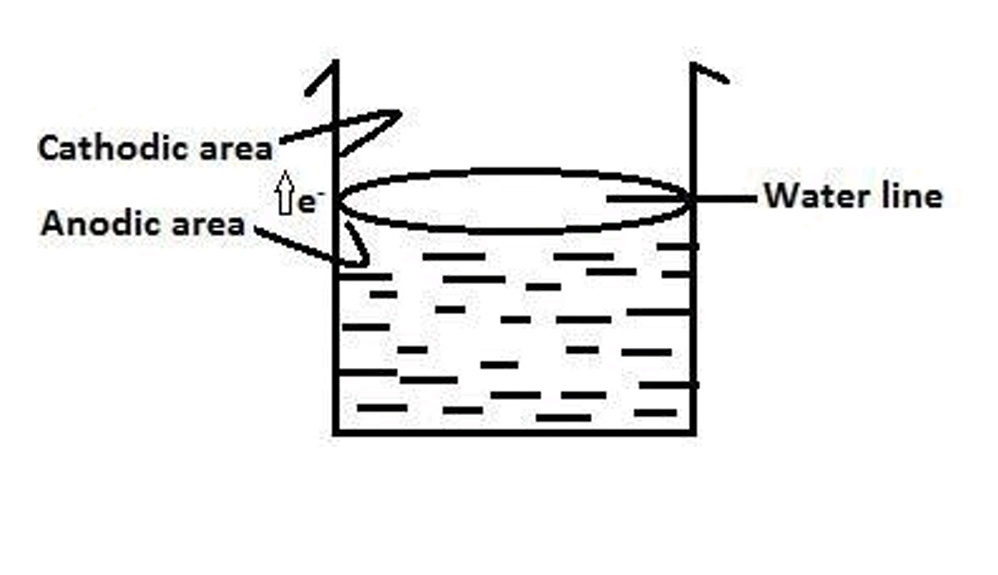
Water line corrosion:
- It is observed in steel or iron water tank partially filled with water. Part of the tank just below water level is exposed to lower concentration of O2 becomes anodic and undergoes corrosion.
- Part of the tank above the water line which is exposed to higher concentration of O2 becomes cathodic and protected from the corrosion.
- More corrosion is observed just below the water line; hence this type is called water line corrosion.